|
Site author Richard Steane
|
The BioTopics website gives access to interactive resource material, developed to support the
learning and teaching of Biology at a variety of levels.
|
|
|
|
Nutrient cycles
Nutrients are recycled within natural ecosystems
Plants and animals need certain chemical elements in order to grow, and within the natural environment some elements are more widely available than others.
They are mainly found chemically combined to other elements, and they may need to be mobilised and then undergo further chemical conversions.
Plant and animal growth depends on their continued supply and the replacement involves a recycling process.
Inorganic or mineral ions from the environment are used as sources of some elements, but they are in short supply and tend to be used up over a period of time.
Organic forms contain these elements in chemical combination with carbon, and other elements such as hydrogen and oxygen. These are the main forms found within living organisms. They are principally produced by plants and taken by animals. These compounds form the basis for the food chains in the ecosystem. They are effectively the biomass of producers and consumers.
In the background, decomposing microorganisms (bacteria and fungi) play an important role in recycling chemical elements such as carbon, nitrogen and phosphorus.
Saprobiotic nutrition
Microorganisms living on dead organisms - plant and animals - are called saprobionts. They gain energy from chemical conversions of organic molecules - principally by oxidation of carbon compounds - and they break down complex chemical components of biological origin into simpler forms. They incorporate some products into their own cells, and release others into the environment. These can then be taken up and re-used by plants.
There are a number of important chemical cycles in Nature.
The carbon cycle is the main one, encompassing photosynthesis and respiration and the interdependence of life on the planet.
It also covers increasingly important modern topics, such as global warming, methane production by cattle and other pollution
The element oxygen also circulates alongside organic compounds in biological transformations and in combination with hydrogen and carbon it undergoes physical and chemical recycling in the environment.
Other elements needed by plants (and animals) have their own cycles: potassium, calcium, iron, magnesium, sulphur (as sulphates), and other trace elements.
And of course there is the water cycle - in which hydrogen oxide moves between different physical states (liquid, gaseous, and solid) mostly without being chemically broken down.
Saprophytic nutrition is a (somewhat outdated) term used to cover fungi and micro-organisms (which used to be classified as plants) growing on rotting organic matter.
The nitrogen cycle
The key inorganic form of nitrogen is the nitrate ion NO3-. It is usually only available in small quantities in soil or water so it is a limiting factor in plant growth. Nitrates are generally very soluble so they can easily be 'washed out' of soils, and into bodies of water.
There is a considerable amount of gaseous nitrogen N2 (78%) in the atmosphere but it is generally not available to living organisms.
This gas - also known as dinitrogen - has a triple bond which is not easily broken.
The nitrogen cycle principally consists of the interconversion between inorganic and organic compounds of nitrogen, notably nitrate being used by plants to create proteins, which are passed on to animals then eventually broken down and recycled by microbes into nitrates.
There is also a branch line (nitrogen fixation) by which atmospheric nitrogen can be helpfully converted into inorganic ions as well as an option running in the opposite direction by which nitrate can be lost from the cycle and converted into nitrogen gas again.
Other inorganic nitrogen compounds
Nitrite ions (NO2-)
are produced from the chemical reduction of nitrates.
[Click to
see/ hide some information
about nitrites in food]
Nitrites have been found to be converted by cooking into nitrosamines which are carcinogenic, but modern meat curing practices using lower concentrations of nitrites in conjunction with ascorbic acid (vitamin C) have reduced the risk considerably.
Ammonia (NH3)
is a gas with a very distinctive pungent smell, which dissolves in water to give an alkaline solution (ammonium hydroxide - NH
4OH)
Ammonium ions (NH4+)
are effectively another stage of reduction on from nitrites.
They are (also) formed when ammonia is neutralised (by an acid).
Ammonium nitrate (NH
4NO
3) supplies nitrogen in anionic and cationic forms, and has been used as a fertiliser.
Oxides of nitrogen (Noxes)
There are a number of gases that are compounds of nitrogen and oxygen. These may be produced by industrial processes and transport. At low concentrations they can can irritate eyes, nose, throat and lungs, possibly leading to coughing, shortness of breath, tiredness and nausea and at higher concentrations they can cause spasms and swelling of tissues in the throat and upper respiratory tract. They can be a special burden to asthmatics.
Car exhausts are generally treated with catalytic converters to reduce their concentration in the air.
Two of the most toxicologically significant compounds are nitric oxide (NO) and nitrogen dioxide (NO
2). Other gases belonging to this group are of environmental importance: nitrogen monoxide (or nitrous oxide, N
2O) [which has a global warming potential of about 300 times greater than carbon dioxide and contributes 6% of planetary warming due to greenhouse gases], and nitrogen pentoxide (NO
5).
Organic nitrogen compounds in plants
Nitrates (or ammonium salts) are taken up by plant roots and passed into the xylem, then delivered to growing cells.
Here nitrogenous ions are converted into ammonia and reacted with carbon compounds (most of which are intermediates in glycolysis and the Krebs cycle) to form
amino acids.
These amino acids can then be built up into
plant proteins as cells grow.
Nitrogen is also used to synthesise
nitrogenous bases (A, C, G, U, T etc) of nucleic acids RNA and DNA.
Within water in rivers, lakes and the sea the same processes occur but the principal organisms are cyanobacteria and algae, which constitute phytoplankton and contribute greatly to environmental processes, not least photosynthesis, with the release of oxygen. Photosynthesis is confined to the upper layer of the sea because light only penetrates for a few metres (perhaps down to 200m), and inorganic nitrogen compounds are consequently incorporated into proteins within cells here. In the deeper ocean, inorganic nitrogen compounds build up but currents bring them to the surface to be used by phytoplankton.
The tiny marine cyanobacterium
Prochlorococcus, discovered in 1986, accounts for more than half of the photosynthesis of the open ocean.
Nitrogen-fixing cyanobacteria in the sea effectively cut out several of the boxes in the nitrogen cycle flow chart below.
These producers (represented by the green box below) are then consumed by zooplankton, so they contribute nitrogen compounds to the aquatic food chain.
Organic nitrogen compounds in animals
Animals cannot
synthesise amino acids, the basic subunits of proteins. They obtain protein by eating plants (or other animals that eat plants). Plant proteins are digested and broken down into amino acids, then absorbed into the blood stream before being taken to growing cells where they are re-assembled into animal proteins. So-called
essential amino acids cannot be produced by modification of molecular structure using other non essential amino acids.
Similarly, they break down nucleic acid subunits and reuse them for their own DNA and RNA.
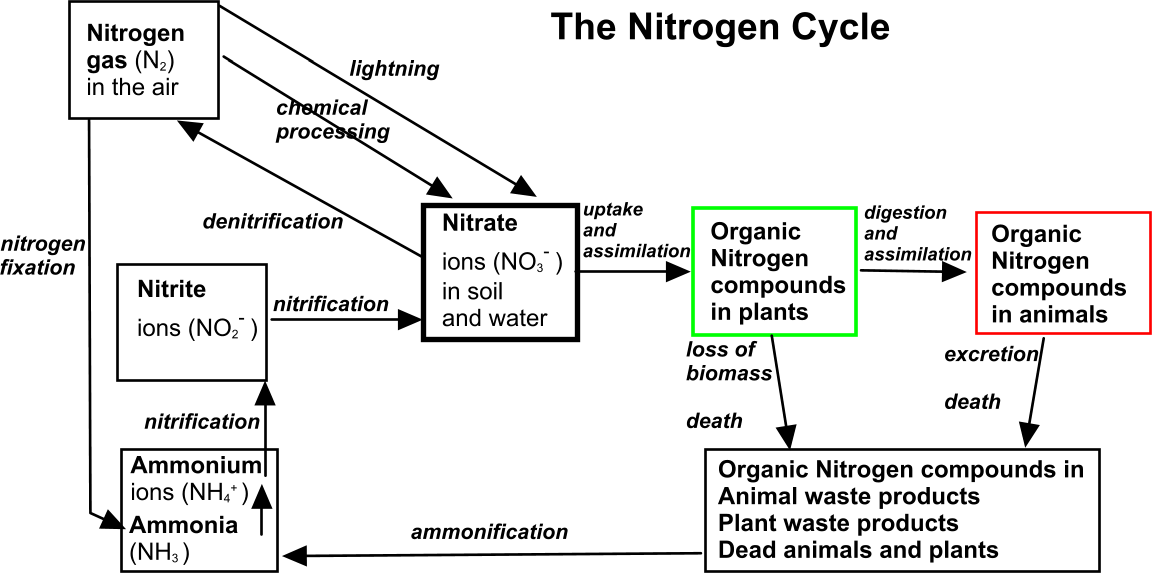
Decomposition of organic nitrogen compounds in plant and animal wastes, and dead plants and animals
Plants tend to withdraw chemically useful compounds from leaves before they fall, but dead plants at the end of their growing season still contain proteins, nucleic acids and other nitrogenous compounds. Even more concentrated forms of nitrogen are removed from the parent organism when a hedge is trimmed or grass is cut on a lawn. All of these products can be broken down (composted) by the action of micro-organisms - bacteria and fungi. This decomposition process can be fairly slow because the cell walls made of cellulose and lignin (composed of chains of methoxylated derivatives of benzene) are very resistant to enzymic hydrolysis.
Animals perpetually consume more food than they need so they excrete the excess. The main nitrogenous waste material from mammals is urea: CO(NH2)2 , formed in the liver by the breakdown of excess amino acids - a process known as deamination.
Urea leaves the body in urine, dissolved in water.
The breakdown of urea is catalysed by the enzyme urease, producing carbon dioxide and ammonia which may be chemically converted to ammonium ions NH4+ which are soluble. Birds and arthropods produce a related compound uric acid which can be disposed of without much water loss. Aquatic organisms often secrete their nitrogenous waste product - ammonia - into the surrounding water.
Faeces - semi-solid undigested food material - is mostly not an excretory product as it has not been taken into cells of the body, but it still contains chemical compounds of plant and animal origin. It also contains bacteria which were living within the digestive system. As they die, their nitrogen compounds become available to decomposers.
Dead animals also obviously still contain protein and nucleic acids. Not only are these more concentrated than in plants, but there is less resistant covering material, so animal wastes are broken down much more rapidly than plant wastes. Chemical breakdown of amino acids in living and dead organisms can produce foul-smelling organic amine compounds. Putrescine, cadaverine and spermine are well known examples, with descriptive names!
Whether it occurs on the woodland floor, the garden compost heap, the sewage works or inside the coffin, the decomposition of previously living material is generally carried out by bacteria and fungi.
These micro-organisms produce digestive enzymes which they release onto their substrate, causing extracellular digestion. The soluble digestion products can diffuse in all directions and at least part can be taken up and used by the micro-organisms for their metabolic processes: respiration and growth. This is called saprotrophic nutrition.
The role of bacteria in the nitrogen cycle
Bacteria are involved in several chemical conversion processes within the nitrogen cycle
Ammonification
Saprobionts such as bacteria (and fungi) act as decomposers.
They carry out extracellular digestion of plant and animal wastes, using some of the organic compounds as respiratory substrates to power their own biological processes. Organic compounds are thus converted into carbon dioxide and water.
A secondary part of this involves the conversion of organic nitrogen (protein/DNA etc) into ammonia (NH3) which in a damp environment is quickly chemically converted into ammonium ions (NH4+).
Nitrification
This is the conversion of other nitrogenous compounds into
nitrate ions (NO3-) , which represent a form of nitrogen that can be readily
absorbed by green plants - the producers in an ecosystem.
Nitrification is essentially a
two-stage oxidation process:
- Ammonium ions (NH4+) are turned into nitrite ions (NO2-) by nitrifying bacteria.
- Nitrite ions are turned into nitrate ions (NO3-) by other nitrifying bacteria.
These bacteria respire aerobically, so nitrification occurs in well-ventilated soils and is inhibited in waterlogged soils.
Nitrosomonas spp are chemoautotrophic bacteria that carry out the first stage of nitrification in soil.
In contrast to the normal process of photosynthesis, they use energy gained through the oxidation of ammonium ions to fix gaseous carbon dioxide into organic molecules.
Nitrobacter spp carry out the second stage of nitrification in soil.
Nitrospira inopinata is a Comammox (COMplete AMMonia OXidiser) - an organism that can both convert ammonia into nitrite and nitrite into nitrate
Nitrogen fixation
This term applies to the conversion of atmospheric nitrogen gas (N2) into soluble nitrogen compounds such as ammonia, and ions like ammonium and nitrates.
Interestingly the term fixation was originally used in an alchemical sense to cover a 'process in which a volatile spirit or essence was reduced or converted to a permanent bodily form'.
[Concise Oxford Dictionary]
Non-biological nitrogen fixation
includes the activity of lightning in thunderstorms, which gives enough energy for nitrogen gas and oxygen to combine forming nitrogen oxides, mostly nitrogen dioxide (NO2). This reacts with rain and falls as (dilute) nitric acid HNO3, which produces nitrate ions (NO3-).
Industrial nitrogen fixation by the Haber process produces ammonia which can then be converted into other nitrogen compounds that can be used as agricultural and horticultural fertilisers.
Biological nitrogen fixation
This process involves various types of bacteria (and
cyanobacteria) which may be
free-living in soil or
symbiotic, generally living within structures such as root nodules of leguminous plants. The term symbiosis covers mutualistic interactions between organisms of two different species, in which each organism benefits from the interaction in some way.
Nitrogen-fixing bacteria produce a
nitrogenase enzyme that combines gaseous nitrogen N
2 with hydrogen to produce ammonia NH
3.
This reaction is powered by ATP and it involves transfer of hydrogen (ions) and electrons from electron chain cofactors.
Nitrogenase is inactivated by oxygen.
In Biology, not all vegetables are legumes
The leguminosae (aka Fabaceae) is the name given to a large family of flowering plants which have fruits called legumes.
Examples are peas, beans (especially soya) and lentils, and 'monkey nuts'. These have characteristic pods containing seeds often used for (human and animal) food, as they are reasonably high in protein.
Root nodules of Clover
 https://lizzieharper.co.uk/
https://lizzieharper.co.uk/
Several species are important for animal fodder:
alfalfa (lucerne),
Medicago sativa
white clover,
Trifolium repens,
red clover,
Trifolium pratense.
All of these species have symbiotic associations with nitrogen-fixing bacteria within nodules on their roots, so they can grow in soils which do not contain much nitrates, and they produce crops which are fairly high in protein, as well as raising soil soluble nitrogen when they die and become decomposed.
While the plant is growing, the symbiotic bacteria produce ammonia which is taken up by the plant, converted into ammonium ions and passed around the plant in the xylem. Most of the nitrogen is combined with organic compounds produced by photosynthesis in the upper parts of the plant. This is turned into amino acids for the production of proteins and organic bases for the production of DNA and RNA.
Some simple carbon compounds are passed between the plant and the bacteria in the root nodules.
Other symbiotic associations
Azolla is a small floating fern, often found growing on the surface of water in rice paddy fields. Within its leaves are cavities in which the cyanobacterium
Anabaena grows. Some of the cells of
Anabaena carry out photosynthesis, but some thick-walled cells called heterocysts do not contain chlorophyll and carry out nitrogen fixation in the resulting anaerobic conditions.
Suggest how the features of the heterocysts improve the efficiency of the process of nitrogen fixation.
>
Thick walls exclude oxygen
>
Produced by photosynthetic cells (of fern and Anabaena)
>
Contain no chlorophyll so do not photosynthesise/do not produce oxygen
>
Oxygen would inhibit nitrogen fixation process
Explain how ploughing the fern plants into the soil results in an
improvement in the growth of the rice crop grown in these fields
>
Decomposers/bacteria/fungi/saprobionts (in fields)convert protein/organic nitrogen (in cells of fern) into ammonium ions / ammonia
>
Ammonium ions /ammonia converted to nitrite then nitrite converted to nitrate
( Just 1 mark for NH3/NH4+ → NO3-)
>
by nitrifying bacteria
>
Nitrate used to form protein / amino acids in rice
>
Link between application of fern and protein/cells of rice
>
Decomposers respire carbohydrates etc and release CO2 - used in photosynthesis by rice
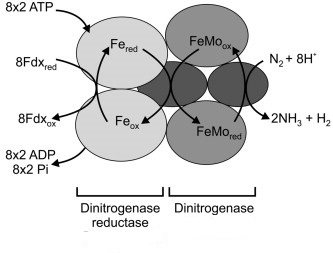
The reduction of nitrogen
The nitrogenase enzyme consists of several sub-units, composed of iron-sulphide complexes and iron-molybdenum complexes.
These are also known as dinitrogenase reductase (a dimer) and dinitrogenase (a tetramer).
The addition of hydrogen to nitrogen occurs in several stages:
pairs of electrons are passed from ferredoxin to the iron-sulphide complex which is activated by ATP
and becomes closer to the iron-molybdenum complex, which is the site of reduction of nitrogen, accepting hydrogen ions and electrons.
This causes the reduction of N
2 to (NH)
2, then to (NH
2)
2 and finally to 2 NH
3
Overall, the change is given by this reaction;
N2 + 8H+ + 16 ATP + 8e- → 2NH3 +H2 + 16ADP + 16 P i
The establishment of root nodules
Rhizobium (leguminosarum) is the bacterial species commonly referred to in symbiotic relationships with leguminous plants.
It enters the plant roots by a number of steps - sometimes described as invading or infecting the plant.
Plant roots release flavonoids which attract
Rhizobium bacteria in the soil, and they enter root hairs.
The bacteria produce signaling molecules called nodulation factors (
Nod factors) which cause root hairs to curl, and allow bacteria to enter cortical cells, where they transform into bacteroids of varying shapes, within compartments enclosed by plant cell membranes.
Interestingly, similar colonisation mechanisms are used by fungi involved in arbuscular mycorrhizal symbiosis.
See below.
Within root nodules, plant cells produce
leg-haemoglobin which absorbs oxygen similarly to its action in animal blood. It is thought that this lowers the concentration of oxygen which inhibits the action of bacterial nitrogenase, but it cannot prevent aerobic respiration which is necessary for the production of ATP.
Nitrogen fixation is also performed by free-living or symbiotic bacteria known as diazotrophs.
In free-living nitrogen fixing bacteria such as
Azotobacter spp., ammonia is assimilated into the amino acid glutamate .
Other nitrogen fixers
Symbiotic
Actinorhizal plants are a group of flowering plants e.g Alder that have the ability to form a symbiotic association with the nitrogen fixing actinobacterium
Frankia, also within root nodules.
These plants are often pioneer species that colonize areas such as sand dunes, moraines (glacial debris) and newly formed soil of volcanic origin, where available nitrogen is scarce.
Certain
Azospirillum species are associated with cereal grasses. This 'associative nitrogen fixation' is important for crops such as rice, wheat, corn, oats, and barley. These bacteria fix appreciable amounts of nitrogen within the rhizosphere - the region of soil in the vicinity of host plant roots, and it is important that the roots are metabolically active, reducing local oxygen levels.
Free-living
Free-living nitrogen fixers in the soil include the cyanobacteria
Anabaena and
Nostoc, and bacterial genera such as
Azotobacter,
Beijerinckia, and
Clostridium.
In the marine environment, nitrogen fixation is seen as a major factor influencing productivity. The filamentous cyanobacterium
Trichodesmium erythraeum
is thought to carry out almost half of the nitrogen fixation in marine systems globally. Unusually, it is able to fix nitrogen in daylight under aerobic conditions without the use of heterocysts. It carries out photosynthesis but converts oxygen to hydrogen peroxide so its nitrogenase is not affected by oxygen.
It occasionally forms blooms ('sea sawdust') and it can rise or fall with the water column by means of gas vesicles in the cells, used to regulate buoyancy.
Its growth rate is limited by iron and phosphate concentrations in the water. Its fibrous mats support a community of small marine organisms.
Photosynthesis occurs using the light-harvesting protein phycoerythrin which is red in colour. In fact it is thought to have given the Red Sea its name.
Denitrification
This is a process in which nitrate (in soil or water) is broken down and reduced, producing nitrogen gas (which passes into the air).
It is brought about by a number of bacterial species, collectively described as denitrifiers.
Denitrification occurs in anaerobic conditions, such as when agricultural ground is waterlogged, as well as in groundwater (emerging from aquifers), wetlands and ocean sediments. Denitrifiers are facultatively anaerobic bacteria, so they can can also exist in aerobic conditions
These bacteria use organic compounds in their environment as respiratory substrates, but if there is no oxygen to act as terminal electron acceptor, nitrate is used instead.
Denitrification effectively reduces the nutrient status of soil and several agricultural practices (ploughing, drainage etc) are carried out to reduce its effect.
Denitrification is a multi stage process:
nitrate (NO3-) → nitrite (NO2-) → nitric oxide (NO) →nitrous oxide (N2O)→ N2
and it may result in undesired consequences such as the release of N2O, which is a greenhouse gas and an ozone-depleting substance.
Example species:
Paracoccus denitrificans aka Micrococcus denitrificans - a gram-negative coccus, but sometimes seen as a rod-shaped bacterium
Denitrifying bacteria have been used in bioreactors to remove nitrates from agriculural and domestic wastewater, and are found in artificial wetland areas set up for the remediation of water from a variety of sources.
The phosphorus cycle
The key form of phosphorus is the
phosphate ion . It is also only available in small quantities so it is a limiting factor in plant growth. Phosphates are fairly soluble but in the presence of calcium ions they can form insoluble calcium phosphate, making them unavailable for plant growth. Of course, calcium phosphate is the basis for animal bones and teeth, which are really quite solid. Bones can take a long time to be broken down, so calcium and phosphorus can be effectively removed from their cycles - think about fossils!
The elemental form of phosphorus is very reactive, so there is none available outside chemical factories.
There is no alternative form of phosphorus in the environment apart from rock phosphates and sediments in bodies of water.
There are, however, a number of important biological chemicals containing phosphate groups attached by covalent bonds. These are well known in respiratory pathways and photosynthesis where they use phosphate groups to transfer chemical energy. ATP is an important biological chemical in these transformations.
ATP is especially important in powering muscular contractions, and phosphocreatine is an accessory compound providing phosphate groups, effectively speeding up the recycling of ATP molecules during exercise.
In metabolic reactions a distinction is sometimes made between organic phosphates, as distinct from the simple inorganic phosphates, often denoted by the code Pi. Organic phosphates are esters formed by condensation reactions between -OH groups on phosphates and -OH groups on sugars, proteins or lipids, leaving a stable covalent -O- bridge.
Phosphate groups form the strong and uniform backbone to the information-carrying molecules DNA and RNA, joining nucleotides by
phosphodiester linkages.
And of course every cell has phosphate groups attached to phospholipids in the external and internal membranes.
In fact, the phosphorus cycle only really consists of the interconversion between inorganic and organic forms of phosphate.
Inorganic phosphate ions, being in solution in water, can move widely. Once taken up by plants they are converted into organic compounds containing phosphate groups which are maintained within cells of plants and animals. Phosphate is recycled when organisms die and are broken down by bacteria and fungi.

Inorganic phosphates - not quite so simple
The simplest form of inorganic phosphate is often written as PO
43-. This is the basic phosphate ion, formed as a conjugate base of phosphoric acid H
3PO
4-, also known as orthophosphoric acid (so it could be called orthophosphate).
Depending on the pH, it may be in equilibrium with other "phosphate" ions.
Aqueous phosphate exists in four forms:
- [a] phosphate ion (PO43-)
- [b] hydrogen phosphate ion (HPO42-)
- [c] dihydrogen phosphate ion (H2PO4-)
- [d] phosphoric acid - trihydrogen phosphate (H3PO4)
Variation in pH causes a shift of equilibrium so that these forms exist as a mixture:
[pH 10-14] a & b
[pH 5-9] b & c
[pH 0-4] c & d
Inside cytoplasm (cytosol) of cells at pH 7.0, phosphate exists as a mixture of 62% H
2PO
4- and 38% HPO
42-, whereas in extracellular fluid (pH 7.4), it is 61% HPO
42- and 39% H
2PO
4-.
Phosphoric acid itself can become polymerised, in a linear way (forming pyrophosphoric acid, tripolyphosphoric acid, or tetrapolyphosphoric acid) or forming rings (metaphosphoric acid), and there are corresponding (poly)phosphates.
The main polyphosphate compound of interest to biologists is the double phosphate pyrophosphate ion PPi which can be formed from ATP:
ATP → AMP+ PPi
The form of phosphate which is found in bones and teeth is more properly called calcium hydroxyapatite, which can become calcium fluoroapatite.
Inorganic phosphate is considered to be an important component of plant mineral "feeds" - which aim to provide balanced amounts of N, P and K (nitrogen, phosphorus and potassium).
Organic phosphates
Glucose 6-phosphate, fructose 6-phosphate, fructose 1,6-bisphosphate and triose phosphate TP are important compounds in glycolysis.
Ribulose 1,5 bisphosphate (RuBP) and glycerate-3 phosphate - GP - are key players in the light independent reactions of photosynthesis but all the compounds in the Calvin cycle contain phosphate groups.
Adenosine triphoshate ATP is different from the above in that it has three phosphate groups, but they are attached to the same point on the molecule, and they can give energy (as well as releasing inorganic phosphate ions, or transfer organic phosphate groups) as a result of hydrolysis.
Mycorrhizae
[myco=fungus, rhiza=root]
Certain fungi growing in the soil can form associations with plant roots.
These can be described as mutualistic symbiosis because fungi gain from sugars produced by plant photosynthesis, and plants can obtain mineral ions and water from the fungus.
Fungi can form structures called mycorrhizae, either on the outside or inside the roots for this transfer.
Fungi can grow for considerable distances in soil. Their mycelium branches in 3 dimensions to colonise an area of ground, effectively searching for anything of organic origin which can be broken down by extracellular digestion and the soluble digestion products are absorbed. The individual microscopic strands - hyphae - give it a large surface area as they ramify over their substrate, hidden under ground. They can also join up - anastomose - with hyphae of other fungal mycelia (of the same species).
As a result they have access to a larger volume of soil than a plant's roots can colonise, and are in a position to absorb both mineral ions and water that a plant needs. They can also play a part in the solubilisation of some nutrients which exist in less accessible forms in soil - perhaps even down to the bedrock
Mycorrhizae are especially important in the absorption of phosphate ions and a variety of other plant mineral nutrients which are not so easily available.
The Fly Agaric
 Amanita muscaria
Amanita muscaria
It is well known that certain types of fungi are associated with trees. For instance the Fly Agaric is usually found near birch trees, although it can be under pine or spruce. The "toadstool" is a sporophore or fruiting body which has the biological function of producing and spreading spores of the fungus, and it is attached to mycelium under the ground, which is itself in contact with the tree roots - forming a mycorrhizal association. Truffles - which are underground fruitbodies - are also associated with tree species by means of mycorrhizae.
The most common type of mycorrhizal association are
arbuscular (AM) endomycorrhizae.
Hyphae of the fungus enter the cortex cells of the plant root. Here they develop into arbuscules, hyphal structures which branch and fan out looking like small trees. Several different fungi, of different species and strains, can be involved.
Mineral ions are exchanged between the fungus and the plant root. The plant produces phosphate transporter proteins on the membrane lining these cells and this enables the uptake of phosphate ions from the fungus.
Using radiolabelled carbon compounds, it has been shown that there is a correlation between the amount of carbohydrate provided by the plant to the fungus and the amount of phosphate supplied to the plant in this exchange, and this may be used by the plants to reward more 'generous' fungal partners..
Several tree species important for timber have
ectomycorrhizal associtions with fungi.
These form a substantial sheath of fungal mycelium around the absorbing root, linked to an extensive network of intercellular hyphae penetrating between epidermal and cortical cells, called the Hartig net.
Heathers and other heathland species also have mycorrhizal associations which support them in growing on barren and infertile land - essentially a man-made habitat.
It has been found that almost all orchids are reliant upon mycorrhizal fungi at some point in their life cycle.
The answer lies in the soil
When a farmer grows a crop on a field, the plants extract mineral ions from the soil. As the crop is harvested, and taken away for consumption by humans or animals at some other location, a certain proportion of those mineral ions are removed from the soil in the field. Some parts of the crop plant may remain in or on top of the soil, and farmers often do their best to encourage this to decompose and return some of the mineral nutrients to the soil.
Similarly, animal carcases and meat products contain mineral resources removed from the land.
Seen from another angle, the waste products (faeces and urine) of humans and animals who have consumed this crop represent a resource which would replace minerals lost from the land. Much of it is, however, flushed away and forgotten about.
Farmers do not generally know the detailed mineral composition of their soils, although there are nowadays fairly high-tech methods of scanning quite large areas and identifying sections which are deficient in some way. But they tend to apply 'fertilisers' to ensure that the next crop receives an acceptable supply of minerals. If not, crop yields could be expected to fall each year.
As a soil conservation technique, some farmers advocate minimum tillage or strip tillage which does not disturb (all of) the soil by ploughing or harrowing.
Crop rotation
On a farm or in a garden, it is possible to allocate different zones for the growth of different crop species. Each crop removes certain minerals from the soil, which could add up over time to make soil less fertile. To a certain extent, legumes are an exception. By growing different plant species in each zone in following years the soil's fertility can be maintained or improved.
Different crops also need different soil preparation, and make different contributions to the soil, so a three-or four-course rotation plan is often recommended.
Potatoes are considered good for soils that have not been cultivated for some time, because they break up the soil as they swell up under the ground. They also do best in slightly acidic soils, so manure may be added before planting them. Cucumbers, gourds and courgettes (Family cucurbitaceae) thrive on rich ground, so they could be substituted on the next cycle.
Legumes (peas or beans) increase the amount of nitrogen in the soil.
Brassicas (cabbages, Brussels sprouts etc) require more calcium in the soil, so 'lime' can be spread on the patch before they are planted. It is best not to go directly back to potatoes afterwards.
Green manure is a crop such as mustard or alfalfa which is grown over the winter, then dug in to increase the organic material in the soil which adds to the water-holdinng capacity of the soil. It is sometimes said that it is good to leave the plot "fallow" - i.e. not growing a specific crop, but to allow other species ('weeds'?) to grow, then dig them over when planting the next crop.
With heavy (clay) soils it is normal to dig over the plot and leave it exposed to the winter weather so that freezing and thawing opens up the soil structure.
Onions and 'root crops' are best grown later in the cycle. These grow better in more uniform soil. It is said that carrots become forked and split if they encounter fresh organic matter in the soil.
Root crops include:
Carrots
Celeriac
Celery
Fennel
Parsley
Beetroot
Chard
Spinach
Parsnips
Salsify
Scorzonera
Turnips
Some crops are affected by different pests and diseases so spacing them out in time means that these are not passed on from year to year, and gives the pests and diseases a chance to die off!
Perennials such as rhubarb and asparagus are left in their own space.
Natural and artificial fertilisers
Substances of biological origin which assist plant growth may also be described as organic or natural fertilisers.
On the other hand there are substances of mineral origin which stimulate plant growth, and these are also called inorganic. Because they need some processing they may be called artificial.
But all of these descriptions are debatable.
The three main elements required by plants are nitrogen, phosphorus and potassium, and the N:P:K ratio is an important factor in decisions about the use of fertilisers. Sulphate and magnesium content are also often monitored, and there are a number of other elements required by plants.
All of the main compounds of these elements are fairly soluble in water.
In fact the chemical compositions of natural fertilisers are rather undefined, whereas artificial fertilisers are more reliable: you can read the analysis on the packaging!
Natural fertilisers
Animal faeces and urine can be collected if animals are kept on impermeable surfaces, such as concrete. The slurry resulting from cows, pigs and chickens is usually scraped into storage areas such as underground pits or lagoons, and during storage this material can cause offensive smells, as well as releasing ammonia gas into the air - a consequence of ammonification.
At an appropriate stage in the crop's development this material is spread on the land, perhaps using a dungspreader towed behind a tractor. Tractors generally have large wide tyres to reduce the pressure on ground and the crops they are driven over.
Animal bedding contaminated with their waste products may be subjected to a composting process which changes it into a more manageable form.
This processing has anaerobic and aerobic phases, and the stacking and mixing are aimed at encouraging the growth of certain micro-organisms.
Mushroom compost is an example of this, and after producing a mushroom crop it remains useful as a horticultural soil improver.
Sewage works in the UK produce over 1,000,000 tonnes of sewage sludge: 'biosolids' - of human origin - each year, most of which is used as agricultural fertiliser. This contains nitrates, and phosphates, which are important for plant growth. However there have sometimes been cases of contamination with minerals of industrial origin: cadmium, zinc, mercury, chromium, selenium and arsenic, and other potentially harmful substances.
There are a number of regulations covering its use, in relation to timing of crop growth cycles. This is especially for soft fruit, vegetables and potatoes, but not for cereals, grass, sugar beet and oilseed rape.
Certain products of animal origin actually provide fairly concentrated and defined components to soil:
Hoof and horn meal contains about 12% nitrogen and 2% phosphorus. It is a waste product of the meat industry, heat-treated (cooked?) and ground so it does not resemble its original components. It takes some time to break down (by bacterial action) and release these plant nutrients into the soil. It can thus be considered as a slow-release fertiliser.
Blood fish and bone is presumably a by-product of fish-processing. Its NPK rating is generally close to 5.5:8:6.
Bone meal is a product derived from meat processing, and contains phosphorus (bone being principally calcium phosphate) as well as nitrogen (from protein in the bone marrow), so it is said to encourage growth of roots. Some imported products have been found to be infected with anthrax , which is concerning because this anaerobic bacterium produces spores which can remain in soil for years. Its NPK rating is typically 3:9:0.
Natural fertilisers can all come under the 'slow release' heading, which makes them less likely to cause pollution problems in rainy weather.
Taking the urine?
Urine has been used for a number of purposes. Within greenhouses it was customary to provide bottles in order to to collect urine from workers. After several weeks storage, the urea became converted into nitrates and it was used it as fertiliser for plants.
And there was also a well-established cottage industry collecting human urine for the production of saltpetre, potassium nitrate, for the production of gunpowder. See also below
Urea - a compound that changed our thinking about the chemistry of life
Urea, also known as carbamide, is an organic compound in the original sense that it typically originates from living organisms - mostly mammals.
It has the formula CO(NH2)2 and it is an organic compound in the more modern sense that its molecular structure is based on the element carbon.
The German chemist Friedrich Wöhler discovered in 1828 that urea can be produced from the inorganic material ammonium cyanate NH4(OCN).
He wrote to Berzelius
"I can no longer hold my chemical water. I must tell you that I can make urea without the use of kidneys of any animal, be it man or dog."
This discovery was considered to be a refutation of vitalism, the unscientific notion that living things are alive because of some special "vital force".
But then the term organic was promoted by people who are opposed to the use of 'artificial' chemicals in the growing of plants and animals for food and other products ....
Artificial fertilisers
Fertilisers containing nitrogen can be manufactured in chemical plants using the Haber process for the production of ammonia.
Although one of the raw materials - nitrogen gas - is freely available, it is a highly energy-demanding process, for the production of hydrogen from methane, and heating the reaction vessel, as well as pressurising it. Thus large amounts of fossil fuels are used.
Nitrates are produced by the Ostwalt process which converts ammonia into nitric oxide, then into nitrogen dioxide which reacts with water to give dilute nitric acid.
Ammonia and nitric acid each need to be neutralised before use on the land.
'Nitrochalk' (Calcium Ammonium Nitrate) is a more manageable product.
Phosphorus compounds for use in fertilisers are obtained from phosphate rocks originally formed from the deposition of phosphates in an ancient marine environment. They are surface-mined from sedimentary deposits in China, the Middle East, northern Africa and the United States, so there is a background transport cost as well as environmental degradation at source. These ores are are not very soluble, so they need to be reacted with acids - usually sulphuric, then the phosphoric acid is neutralised with ammonia to give ammonium phosphate.
Potassium is generally provided by the salts potassium chloride or potassium sulphate which are mined from underground deposits of evaporite - originally derived from seawater.
This also contains sodium chloride and clay minerals which can be separated out from solution by a crystallisation process involving the addition of amine reagents which coat only the potassium chloride crystals and make them float to the surface to be skimmed off when air is pumped in.
At present there are plans to mine the mineral polyhalite from underground in northern England, in fact beneath the North York Moors National Park. It is expected to extract 10-20 million tonnes per year, which is equivalent to the current world demand for this mineral.
It has been described as a 'low-chloride potash fertilizer with additional magnesium and sulphur components'.
NPK fertilisers consist of mixtures of mineral salts such as potassium and ammonium nitrate and phosphate. These are supplied as granules, crystals or powder to be spread on the land or dissolved as a concentrate to be diluted before application to plants in pots and grow-bags.
These products are advertised with ratios of N:P:K, to show the contributions of these three main elements. Often they give details of the analysis methods used in relation to chemical standards.
For example, P2O5 - phosphorus pentoxide and K2O - potassium oxide - are often mentioned, although they are unlikly to be actually present, and 'ammoniacal nitrogen' refers to ammonium salts rather than nitrate.
Many granular solid garden fertilisers are based on a product National Growmore piloted in the last war to encourage the public to grow vegetable produce and assist in the war effort - with the slogan 'Dig for Victory'!
Typically this has a NPK value of 7-7-7. Its recommended application rate is 70g per square metre, and it is usually raked lightly into the soil surface.
'Liquid tomato food' has a NPK value of close to 4-3-8 or 4-5-9 but it is generally diluted more than 200x or 500x..
Many branded plant fertilisers are available, providing not only N, P, and K but also very small quantities of a number of other trace elements, which are cheap to supply.
Mineral ions in the artificial fertiliser category are much more quickly available than in natural fertilisers. They can bring a 'quick green-up' to crops, lawns and garden plants, but they can be more easily washed out of soil, causing a number of environmental problems.
Weapons take priority
For centuries, nitrates were used in the production of gunpowder and other explosives in Europe and America.
Saltpetre (potassium nitrate) and chile saltpetre (sodium nitrate) were imported from India and other parts of the world, and control of supply of these minerals played a large part in a number of military campaigns.
A secondary use of saltpetre was as an agricultural fertiliser, providing both nitrogen and potassium.
More recently, industrially produced ammonium nitrate has been used for both purposes.
In agriculture it is seen as a high-nitrogen fertilizer, providing both NH4+ and NO3-.
Mixed with fuel oil (FO), it is used to make ANFO, a popular industrial explosive which accounts for 80% of explosives used in North America, principally
in mining, quarrying, and civil construction.
It has also been used in improvised explosive devices in a number of countries and its manufacture and storage are subject to a number of controls.
Hydroponic growing
Plants can be grown in soil-less systems where all their mineral nutrients are provided by solutions of artificial fertilisers, pumped from bulk supply tanks. These can be finely controlled to maximise growth and cropping. These typically have two supply tanks: one containing calcium nitrate (along with other components), and the other containing potassium phosphate (and other components) which are diluted when they mix with the circulating liquid.
Why are calcium and phosphate provided in different solutions?
>
Because they can form calcium phosphate which is insoluble
Environmental issues arising from the use of fertilisers
The production and storage of fertilisers can have a number of impacts on the environment.
Mining and transport of minerals can affect the land and air, not necessarily where they are initially produced.
Storage of products of biological origin can present cause offensive odours and on the days when manure or sewage sludge is spread, everyone in the countryside is aware. There can be major pollution problems if leakage occurs into watercourses.
Leaching
The loss of water-soluble plant nutrients from the soil is inevitable when a large amount of rain falls, or ground is flooded, perhaps by water from higher areas. Nitrates and phosphates are some of the most soluble salts, so they are likely to be in the run-off water leaving agricultural ground.
But it depends on the nature of the soil; clay soils retain minerals better on account of their finer particle structure which absorbs water, as well as the chemical adsorption of cations by clay minerals whereas sandier soils drain quickly and do not retain mineral ions to such an extent. Phosphate can be adsorbed by iron and aluminium oxides which are common in soil, so it is not quite as easily washed out of soil as nitrate is.
The stage of development of the crop can have an effect; cereal crops grow side shoots - a process known as tillering - so they act as a physical barrier to water movement.
And of course there is a distinction between natural and artificial fertilisers. Natural products are bulkier and slightly spongey so they hold on to water and ions within their structure. They still require bacterial decomposition to release all of the elements they contain so they are described as slow-release fertilisers.
Purely mineral products are almost instantly accessible to plant via their roots but whatever is in the soil can be lost by leaching.
This can cause a problem for a farmer because crops do not receive as much mineral nutrition as expected, as well as problems accessing the waterlogged land.
But the lost nitrates and phosphates will have effects on the environment they flow into.
Washday problems
Laundry detergents ('washing powders') used to contain phosphates because they helped to 'soften the water' by extracting calcium and magnesium ions. At the end of the wash cycle, the phosphates were discharged into the water which flowed down the drain then through treatment works and into rivers and the sea.
This caused eutrophication and algal blooms (see below).
Largely as a result of pollution of the Great Lakes and other water courses, the USA banned phosphate-containing laundry detergents and similar measures were enacted in other parts of the world in recent years. Restrictions have also been placed on dishwashing detergents.
Eutrophication
This is the process through which water in streams, rivers, lakes or sections of the sea becomes overloaded with nutrient-rich water. There will always be a certain amount of mineral nutrients in these bodies of water, as well as dissolved oxygen and carbon dioxide, together with a spectrum of simple plants and bacteria. Upland water is often described as oligotrophic in that it does not contain much in the way of minerals which plants need for growth.
When leaching (of nitrates and phosphates) occurs, simple aquatic plants will grow more and multiply in number, possibly resulting in large blooms of algae and cyanobacteria (which are still often called blue-green algae). Water may be coloured by these organisms and chemicals released into the water may be toxic to humans or animals.
Filamentous algae are also encouraged to grow along the edges of flowing water and in shallow areas, especially near to the sources of outflow.
As the water becomes deeper and the density of suspended (mostly single cell) plants becomes greater, less light can enter the water and the simple plant cells die, and sink. Bacteria in the water break them down and respire using their organic matter as an energy source. In so doing, they use up all the oxygen
which was in the water. This results in the death of all the aerobically respiring organisms in the water - from arthropods to fish.
Dead zones are low-oxygen, or hypoxic, areas in the world's oceans and lakes. These are well known in some areas such as the Gulf of Mexico and the Chesapeake Bay, on the East Coast of the United States.

Bodies of water (especially those surrounded by farmland) are often declared out of use during hot sunny weather.
Other related topics on this site
(also accessible from the drop-down menu above)
Similar level
Inorganic ions - Phosphate ions at the end
Skeletal muscle
Structure of Nucleic Acids (DNA and RNA)
Adenosine triphosphate
Slightly different background
Vitamins and Minerals - from a human perpective
Plant Mineral Nutrition - Inorganic & organic fertilisers and their downside at the end
Lower level
CYCLES IN THE ECOSYSTEM Carbon and Nitrogen Cycles but lots of relevant tutorial questions
Web references
Biological Nitrogen Fixation
Dinitrogenase Reductase
The phosphorus cycle NZ angle
Polyphosphate fertilizers increased maize (Zea mays L.) P, Fe, Zn, and Mn uptake by decreasing P fixation and mobilizing microelements in calcareous soil - Say no more
Arbuscular mycorrhizas from David Moore's World of Fungi: where mycology starts - links to other types of mycorrhiza
Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis
A Phosphate Transporter from Medicago truncatula Involved in the Acquisition of Phosphate Released by Arbuscular Mycorrhizal Fungi
Strange but True: The Largest Organism on Earth Is a Fungus - A shame it's a parasite
What farmers need to do to use sewage sludge safely - information for farmers
Will polyhalite disrupt the fertilizer industry?
Potash on a megascale

 https://lizzieharper.co.uk/
https://lizzieharper.co.uk/



 Amanita muscaria
Amanita muscaria

